Certain adults are at increased risk of complications or susceptible to vaccine preventable diseases if they have not previously received the vaccination and are in contact with individuals who have the infection. Vaccinations recommended under the NAIS aim to prevent such infections among susceptible individuals and reduce complications, morbidity, and mortality.2
The NAIS was developed based on international best practices and the recommendations of the Expert Committee on Immunisation (ECI). Considerations include:
Local disease burden;
Age, pre-existing medical conditions, vaccination history;
Vaccine safety, efficacy and cost effectiveness of the vaccines in preventing infections among susceptible individuals and reducing complications, morbidity and mortality.
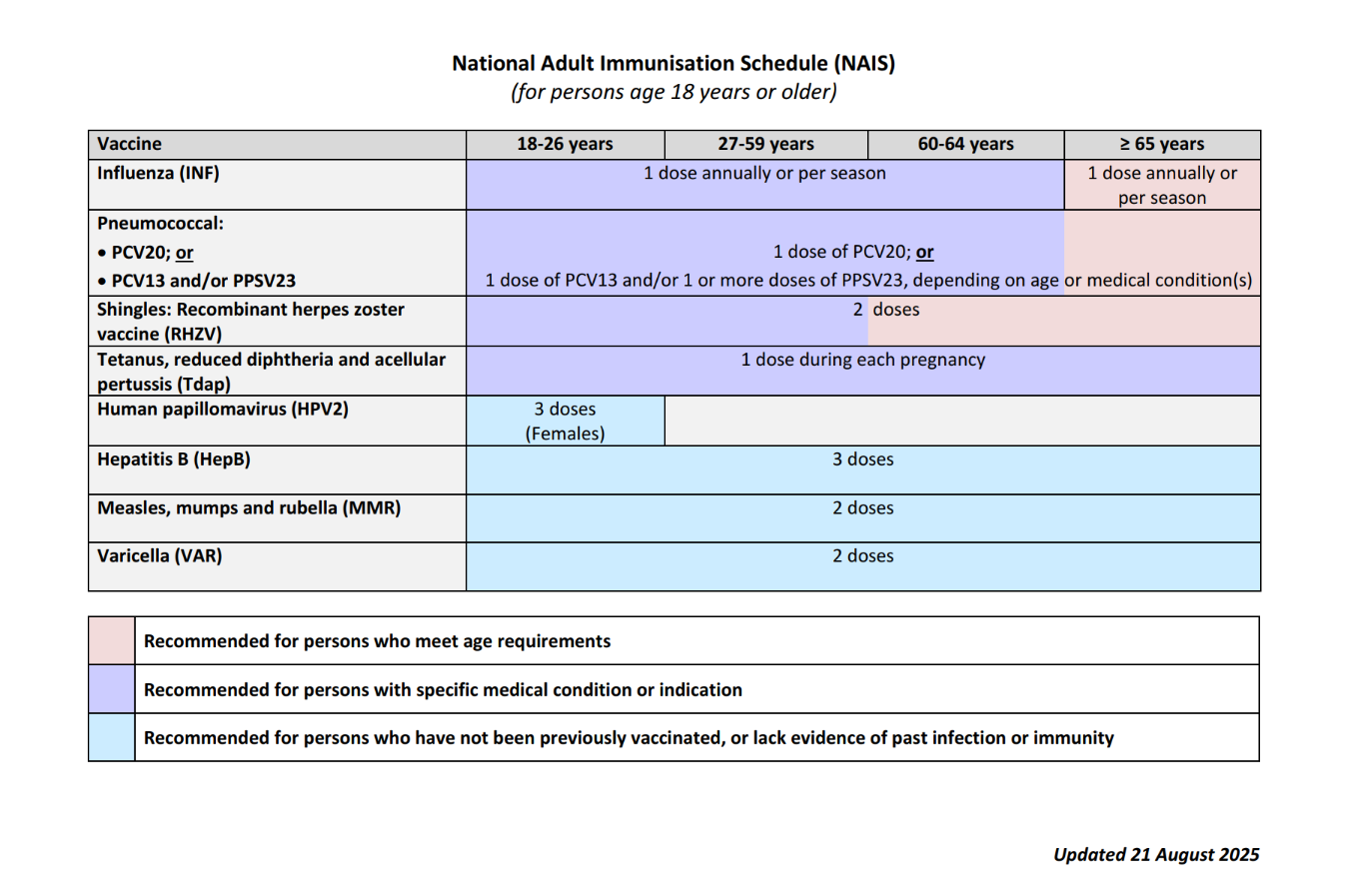
The NAIS is summarised in
Table 1 below.1
Table 1: National Adult Immunisation Schedule
COVID-19 Vaccinations
The COVID-19 vaccination recommendations for 2025/2026 remain unchanged from the 2024/2025 recommendations, as the vaccination continues to be recommended for individuals at
increased risk of severe COVID-19. The summarised recommendations are shown in
Table 2.
Table 2: Summary of 2025/2026 COVID-19 Vaccine Recommendations
Vaccination recommendation
| Number of doses
| Interval between doses
|
(a) Initial doses (for unvaccinated persons) |
i. Recommended for higher-risk groups:
- Individuals aged 60 years and above
- Medically vulnerable individuals aged 6 months and above
- Residents of aged care facilities
| Age 6 months – 4 years: 2 doses1, 2 (eight weeks apart) Age ≥ 5yrs: 1 dose2
1(a) If the child has had a documented COVID-19 infection, providers may at their clinical discretion, and in discussion with parents, decide to administer one initial vaccine dose instead of two. (b) Children whose second dose is due on or after turning 5 years old, should still receive the second dose at the dosage for a 5-year-old after the recommended interval from the first dose.
2Immunocompromised individuals (incl. those previously immunoablated) are recommended a 3-dose enhanced primary series.
|
ii.
Allowed for vaccination: Other individuals aged 6 months and above
|
(b) Additional doses (for vaccinated persons) |
iii.
Recommended for vaccination: Same as
(a)(i)
| One additional dose
| One year (and at least five months from last vaccine dose)
|
iv.
Encouraged for vaccination:
- Healthcare workers
- Persons living/working with medically vulnerable individuals
|
v.
Allowed for vaccination: Same as
(a)(ii)
|
Refer to
MOH Circular No.
67/2025 for more detailed clinical guidance on the COVID-19 vaccination.
Other Vaccinations
For the use of other vaccinations (e.g. yellow fever for travel to endemic areas, meningococcal for Haj pilgrimage), please refer to the Package Insert (PI), available on the Register of Therapeutic Products (under E-services, Infosearch) on the
Health Sciences Authority (HSA) website. The National Immunisation Registry (NIR) only accepts vaccination records for those under the NAIS.
Administering Vaccinations
Healthcare providers should ensure the following during the administration of any vaccine:
The vaccine is being given to the correct patient.
The vaccine is given at the appropriate time, i.e. appropriate age and interval.
The correct vaccine and diluent are given to the patient.
The appropriate dose has been measured.
The vaccine is being administered via the correct route and technique e.g. subcutaneously vs intramuscularly.
The vaccine is administered at the correct site e.g. deltoid vs anterolateral thigh.
The vaccination details should be documented in NIR and the clinic's Electronic Medical Records (EMR) accurately with the type of vaccine, diluent, dose, batch, expiry, date and time administered, route, and consent.
After the administration of vaccines, patients should be observed for any immediate post-vaccination adverse effects. Patients should also be advised to monitor for possible side effects of the vaccine once they have been discharged. Details on NAIS for the public is available at Ministry of Health Singapore | Vaccinations

